This post is also available in:
Español
Português
The IBU (International Bitterness Unit) index is one of the most vital beer statistics that is frequently manipulated and misunderstood by brewers and the general public, respectively, primarily for marketing purposes.
The first thing to understand is that, from the perspective of a chemist and a professional brewer, this bitterness unit does not refer to the “perceived” flavor in the beer, but rather to the levels of certain bitter compounds dissolved in the liquid.
Contenido
What are IBUs?
The IBU is a standardized scale that accurately describes the bitter content of beer as a direct measure of the concentration of isohumulones, the chemical compounds responsible for bitterness in beer.
The IBU scale represents parts per million (ppm) of isohumulones in the sample, not the perceived bitter taste, which is often balanced or masked by malts and other ingredients.
In essence, the calculated IBU value is not entirely representative of the bitter taste perceived by the consumer in the final product.
The IBU Bitterness Scale
The IBU scale starts at 0 and, in theory, has no upper limit. However, since the compounds that produce bitterness in beer are only water-soluble, there is a practical limit to the number of molecules that can be contained in a given volume of liquid.
Additionally, studies on human taste perception suggest that our bitter taste receptors have a saturation limit, though the exact degree is still debated.
What Causes Bitterness in Beer?
The bitter taste in beer comes from the hops used during production. During the brewing process, the alpha acids in hops are isomerized into iso-alpha acids, or isohumulones, which ultimately give beer its bitter flavor.
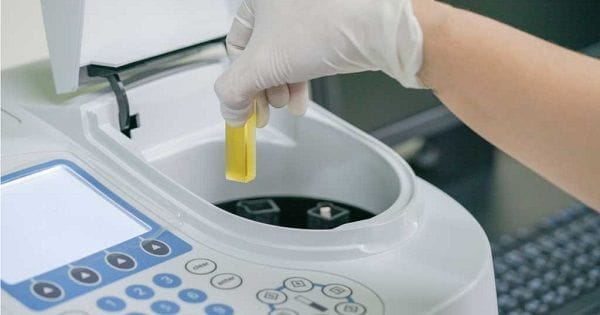
What is a Spectrophotometer?
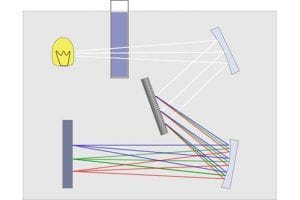
A spectrophotometer is an instrument used in chemical analysis that measures, based on wavelength, the relationship between values of the same photometric magnitude relative to two beams of radiation, as well as the concentration or chemical reactions measured in a sample.
How to Calculate IBU in a Laboratory
The measurement of IBU was standardized in 1964 by the American Society of Brewing Chemists (ASBC), a professional organization that deals with virtually all scientific aspects of beer brewing.
To measure IBU, samples of the beer are first taken and placed in conical vials, to which a small amount of hydrochloric acid and an organic solvent called isooctane, also known as 2,2,4-trimethylpentane, are added.
Isooctane and water are immiscible, meaning they do not mix and remain separated into layers.
Isohumulones, on the other hand, are slightly soluble in water under normal conditions, but become much less soluble once the beer is acidified, which drives the isohumulones out of the beer and into the isooctane.
The conical vials are then placed on a shaker for 15 minutes until the liquids form an emulsion, where the increased surface area of contact between the two liquids facilitates the transfer of isohumulones from one to the other.
Finally, the vials are centrifuged for at least seven minutes to break the emulsion.
Wavelengths
Next, a portion of the isooctane (now enriched with isohumulones) is extracted and placed in a glass, quartz, or plastic container, as long as it is optically transparent at the necessary wavelengths.
The container is placed in a UV-Vis spectrophotometer (UV = ultraviolet; Vis = visible), and a beam of white light passes through the sample toward an optical sensor located at the other end.
The isohumulones in solution in the cuvette will primarily absorb specific wavelengths, in this case, 275 nanometers. Then, once the absorbance number is obtained, only a quick and simple final calculation is needed to reveal the actual IBU value of the beer.
No se encontraron productos.